A research team led by Ng Kar Wei and Wang Shuangpeng, two associate professors at the Institute of Applied Physics and Materials Engineering (IAPME) of the University of Macau (UM), has proposed a novel electrochromic battery based on a hybrid aqueous electrolyte, which can help to resolve the problem of the short lifespan of traditional electrochromic devices based on aqueous electrolytes and is expected to enable the commercialisation of low-cost, high-safety aqueous electrochromic batteries. The detailed investigation of the intrinsic mechanism for the superior performance is also expected to provide potential guidance for the design of other high-performance electrochemical devices. The research results have been published in ACS Nano, a leading international journal in the field.
Electrochromic technology is considered a key for realising energy-saving green buildings in the near future. Electrochromism refers to the dynamic and reversible adjustment of the optical properties (such as transmittance, reflectivity and colour) of specially designed nano-films via electrically triggered redox reactions. Smart windows based on this technology can selectively absorb or reflect external heat radiation and prevent internal heat dissipation, thus enabling the effective regulations of indoor room brightness and temperature with very low energy consumption. Since its working principle is similar to that of rechargeable ion batteries, electrochromic devices can serve the purpose of both light regulation and energy storage simultaneously, giving rise to the concept of multifunctional electrochromic battery (ECB). However, most existing ECBs are based on Li-ion organic electrolytes and they exhibit slow response speed and low cycling stability, which limit their commercialisation. Multivalent ion aqueous electrolytes have therefore been recently developed for their better safety and higher conductivity than traditional organic electrolytes. Indeed, notable improvements in response speed and capacity have been demonstrated in aqueous-based ECBs. Nevertheless, owing to the mismatch between the electrochromic nano-films and the aqueous electrolyte, these ECBs can only maintain stable operations in around 1,000 cycles, which is far from meeting the requirement for commercialisation (> 104 stable operation cycles). Therefore, developing a novel architecture with matching electrochromic materials and aqueous electrolytes is crucial for the realisation of practical low-cost, high-performance electrochromic devices.
To achieve the aforementioned goal, the research team has designed a highly compatible ultra-stable multifunctional electrochromic system composed of lithium titanate electrochromic film and Al3+/Zn2+ aqueous electrolyte. The matching between lithium titanate films and various types of aqueous electrolytes has been extensively investigated, and the most optimised system exhibited remarkable electrochromic performances, including fast response speed (4.35 s/7.65 s for bleaching/ colouring), high discharge capacity (151.94 mAh m-2), and record-high cycling stability (>12500 stable operation cycles with zero optical contrast loss). The studies have shown that the high electrochemical activity of Al3+ ions helps promoting the efficient redox reactions of lithium titanate films, thus resulting in excellent optical contrast; Zn2+ ions can suppress the irreversible generation of hydrogen and improve the reversibility of the whole reaction. The synergistic matching between the hybrid Al3+/Zn2+ aqueous electrolyte and the lithium titanate thin film enhances the electrochromic and energy storage performance, making it far superior to the existing aqueous electrochromic batteries based on the traditional tungsten oxide thin films. In particular, its excellent cycling stability makes lithium titanate thin films a competitive next-generation electrochromic and transparent electrode material. These exciting results enable the commercialisation of low-cost, high-performance aqueous multivalent ion electrochromic devices, and also provide alternative design solutions for lithium titanate to be used in other aqueous electrochemical devices.
Prof Ng and Prof Wang are the corresponding authors of the study and the first author of the paper is Wu Zhisheng, a PhD student at IAPME. Other PhD students, namely Lian Zhendong, Yan Shanshan, and Li Jielei, also made important contributions to this study. The project was funded by the Science and Technology Development Fund, Macao SAR (File no: 0038/2019/A1, 199/2017/A3, 0125/2018/A3, and 0071/2019/AMJ). The full version of the paper can be viewed at https://doi.org/10.1021/acsnano.2c06479
澳門大學應用物理及材料工程研究院副教授吳嘉偉和王雙鵬的研究團隊首次提出一種新的電池技術,以水系電解質解決了現有基於水系電解液的傳統電致變色器件存在的壽命短的問題。新技術能使低成本、高安全性的水系電致變色電池商業化成爲可能,並且深入探究其高性能來源的內在機理,有望為其它高性能電化學的器件的設計提供潛在的設計指導。該研究成果已獲國際知名學術期刊《美國化學會—納米》(ACS Nano)刊登。
電致變色技術被視爲未來實現高效綠色節能建築的絕佳策略,因爲它可以通過施加極低的電壓觸發氧化還原反應,表現爲納米薄膜光學性質,例如透過率、反射率、顔色等動態且可逆的調整。基於此技術的智能窗可以選擇性地吸收或反射外界的熱輻射和阻止內部熱逸散,從而以極低的自身能耗實現室內亮度和溫度的有效調節。由於其工作原理與可充電離子電池類似,電致變色器件可以同時用於調光和儲能,多功能電致變色電池(ECB)的概念應運而生。但由於傳統的電致變色器件大多基於鋰離子有機電解質,其較慢的響應速度和較低的循環壽命限制了它們的實際商業化應用。因此近年來,多價態離子水系電解質受到越來越多研究者們的關注。水系電解質相比於有機電解質有著更高的安全性和離子電導率。事實上,基於水系電解質的器件已被證實其顯著提升的響應速度和容量,但由於電致變色納米薄膜和水系電解質之間的不匹配,例如氧化鎢薄膜在酸性電解質中的腐蝕問題。水系電致變色電池只能在大約1000个循環內穩定運行,這遠遠達不到實際商業化的要求(>104個穩定的運行循環)。因此,開發一種電致變色納米薄膜和水系電解質互相匹配的全新架構對於未來低成本,高性能的電致變色器件的商業化至關重要。
有鑒於此,研究團隊設計了由鈦酸鋰電致變色薄膜和Al3+/Zn2+水系電解質組成的高度相容的超穩定多功能電致變色系統。通過系統地研究鈦酸鋰薄膜與不同水系電解質的匹配,得到的最優體系表現出卓越的電致變色性能,包括快速的響應速度(著色/褪色時間為7.65 s和4.35 s),高放電容量(151.94 mAh m-2),和創紀錄的循環壽命(>12500次穩定循環,無明顯光學對比度下降)。研究表明,Al3+離子具有高的電化學活性以促進鈦酸鋰薄膜的有效氧化還原,從而實現優異的光學對比度;Zn2+離子可以抑制不可逆氫氣的產生,提高了反應的可逆性。Al3+/Zn2+混合水系電解質和鈦酸鋰薄膜之間的協同匹配提高了電致變色和儲能性能,使之遠優於基於傳統氧化鎢薄膜的水系電致變色電池。尤其是其卓越的循環穩定性,使得鈦酸鋰薄膜成爲具有競爭力的下一代電致變色和透明電極材料。這些令人興奮的結果使低成本,高性能的水系多價態離子電解質基電致變色器件的商業化成爲可能,同時也為鈦酸鋰用在其他的水系電化學裝提供了潛在的設計依據。
該項研究的通訊作者為吳嘉偉和王雙鵬,論文的第一作者為澳門大學應用物理及材料工程研究院博士生吳智升,澳大博士生連震東、嚴閃閃和李潔蕾也對此課題作出重要貢獻。此項研究由澳門特別行政區科學技術發展基金(檔案編號:0038/2019/A1, 199/2017/A3, 0125/2018/A3, 和 0071/2019/AMJ)資助。全文請瀏覽:https://doi.org/10.1021/acsnano.2c06479
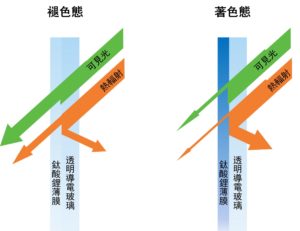
A Schematic Diagram of Electrochromic Smart Windows
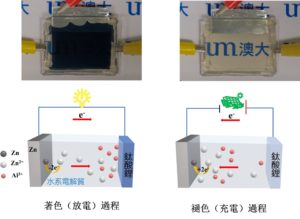
The colouring and bleaching processes of a lithium titanate electrochromic battery and corresponding working principles. The state of energy storage of the device can be visually judged by the change

NG Kar Wei (3rd from left in the back row),
WANG Shuangpeng (5th from left in the back row),
and WU Zhisheng (4th from left in the back row)

